Edx-biomanufacturing学习笔记
Table of Content
Unit 1 Introduction#
Section 1 Introduction##
1.1A Why Biologic Drugs are important?###
1.1B What to expect for the course
1.2 What are biologic drugs?
In the FDA definition, biologic drugs: Proteins, Vaccines, Blood components, Cell therapies, Natural hormones, Pland/animal extracts
Biologics manufactured using recombinant DNA technology: Replace missing function, Selective, Fewer off-target effects
Section 2 Using Cells for manufacturing
###1.3 An introduction to the history of modern biomanufacturing
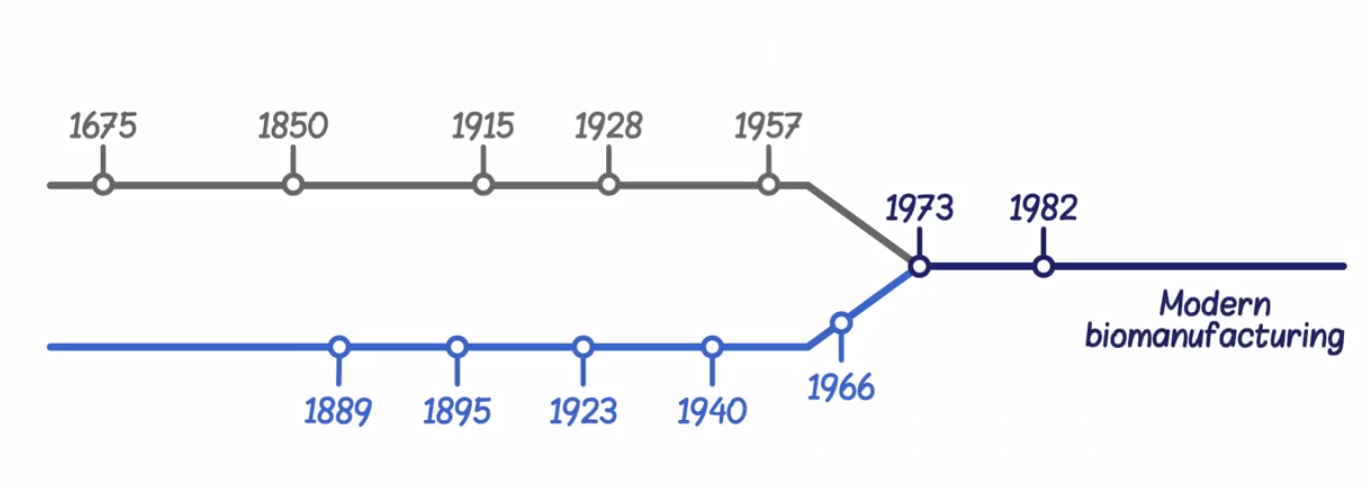
1675, Antonie van Leeuwenhoek, Netherlands, mciroscope
1850, Louis Pasteur, France, alcoholic fermentation (yeast: alcohol; bacteria: lactic acid)
1.4 USING CELLS TO MANUFACTURE CHEMICALS
1915, Wilhelm Connstein and Karl Ludecke, together with Carl Alexander Neuberg and Elsa Reinfurth, German, used yeast to manufacture glycerol (甘油).
1904, Chaim Weizmann, UK, isolated the bacterium, Clostridium acetobutylicum, which could ferment grain to produce butanol (丁醇).
(Cell manufacture chemicals; Aseptic processing = better control)
1.5 USING CELLS TO MANUFACTURE PENICILLIN AND VACCINES
1928, Alexander Fleming, London, identified penicillin, which killed or prevented the growth of a number of different bacterial species.
1939, Howard Florey and Ernst Boris Chain succeeded in isolating penicillin.
1957, Jonas Salk, the production of the polio (骨髓灰质炎) vaccine was the first time mammalian cells were used in manufacturing.
(First, aseptic processing provides better control of the culture and its products.)
(Second, microorganisms can produce safe and effective drugs.)
(Third, genetic mutation and selection can be used to improve cellular productivity.)
(And fourth, mammalian cells can produce therapeutics.)
##Section 3 Protein as therapeutics
###1.6 THE HISTORY OF PROTEINS AS THERAPEUTICS: ANTI-TOXINS
1894, Emil von Behring, German, invented the diphtheria (白喉) anti-toxin
1.7 THE DISCOVERY AND ISOLATION OF INSULIN
1989, Oscar Minkowski and Joseph von Mering found unknown substance in the pancreas, which we now know is insulin, was responsible for regulating sugar levels in the body
1920, Frederick Grant Banting and Charles Herbert Best extracted what they hoped was insulin from the pancreas of dogs
(Challenges: 1. Different sequence; 2. Supply)
1.8 ISOLATION OF PROTEIN THERAPEUTICS FROM PLASMA
1940, Edwin J. Cohn, began to work on isolating Albumin (白蛋白) from blood plasma through a process called fractionation.
1970s, factor III replaced albumin as the most profitable product in the plasma fractionation industry.
(Challenges: 1. Supply; 2. Contamination)
##Section 4 Intro to modern biomanufacturing
1.9 THE BIRTH OF MODERN BIOMANUFACTURING
1973, the recombinant DNA technology
1979 ~ 1981, Genzyme (beta-glucoserebrosidase purified from human placenta, Ceredase, Cerezyme), Genentech (first human insulin produced using recombinant DNA technology)
###1.10 BIOMANUFACTURING IS THE VEHICLE TO DELIVER HIGH-QUALITY BIOLOGICS
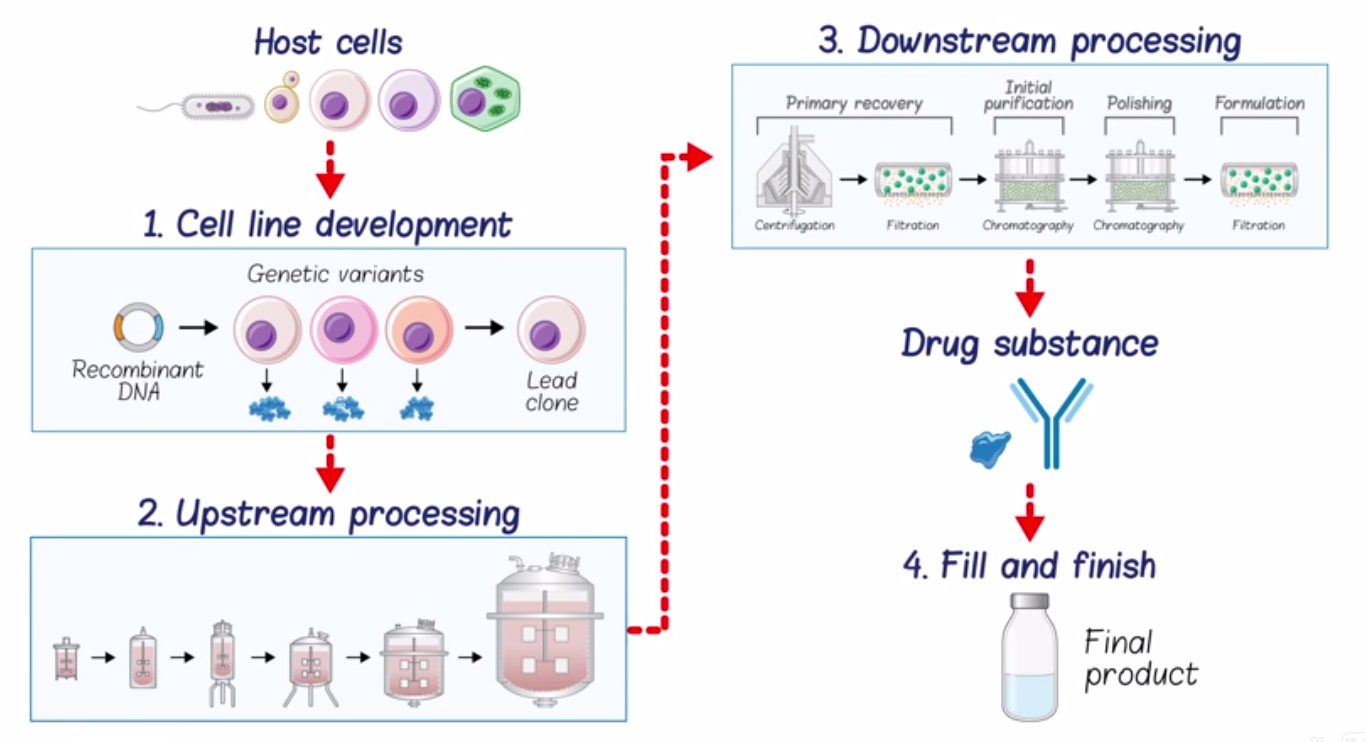
Unit 2 Protein Structure & Examples
Section 1 Introduction to Protein Structure
2.1 Introduction to biologic drugs
| small molecules | Recombinant biologics |
|---|---|
| Aspirin, Penicillin, Ibuprofen | MAb (e.g. Rituximab), insulin |
| 100 - 900 Da | 5,000 - 250,000 Da |
| Chemical systhesis | Cellular synthesis |
| Simple analysis | Complex analysis |
| absorbed through GI tract | parenteral: bypass GI tract |
| mimic compounds; block pathways; unintended interatcions | specific; few off-target effects |
- Drug targets:
- Receptor proteins;
- DNA;
- RNA
- Reduce biologic effectiveness:
- misfolding;
- aggregate
- Parenteral (不经肠的):
- intravenous (静脉) injection;
- intramuscular (肌肉) injection;
- subcutaneous (皮下) injection;
- inhalation (吸入);
- mucosal (粘膜) transmission
- Categories of biotherapeutics:
- Enzymatic or regulatory activity (e.g. Insulin; Imiglucerase for Gaucher’s disease; Erythropoietin for stimulating production of RBCs and anemia (贫血));
- Special targeting activity (e.g. Rituximab for B-cell non-Hodgkin’s lymphomas (淋巴瘤));
- Protein vaccines (e.g. Hepatitis (肝炎) B; Influenza (流感); Human papillomavirus)
###2.2 Introduction to amino acids
- 20 amino acids
- Polar, Uncharged: Serine (Ser, S); Threonine (Thr, T); Asparagine (Asn, N); Glutamine (Gln, Q)
- Polar, negatively charged: Aspartic acide (Asp, D); Glutamic acid (Gln, Q)
- Polar, positively charged: Lysinee (Lys, K); Arginine (Arg, R); Histidine (His, H)
- Hydrophobic: Alanine (Ala, A); Valine (Val, V); Methionine (Met, M); Leucine (Leu, L); Isoleucine (Ile, I); Phenylalanine (Phe, F); Tyrosine (Tyr, Y); Tryptophan (Try, W)
- Special: Glycine (Gly, G); Cysteine (Cys, C); Proline (Pro, P) |Peptides|Proteins| | :——– | ——–:| |<= 40 amino acids| > 40 amino acids|
###2.3 Introduction to Protein Structure
- Protein Structure:
- Primary structure: the amino acid sequence
- Secondary structure: interactions among backbone atoms (α-helix, β-sheet, loop) (hydrogen bone between backbones)
- Tertiary structure: interactions among all atoms in 3D space (bisulfide bonds of cysteines, electrostatic interactions - ionic bonds bewteen opposite charges, hydrogen bone between threonine with water, hydrophobic interactions)
- Quaternary structure: arrangement of multiple protein subunits (monomer, dimer, hexamer)
###2.4 Post translational modifications (PTMs)
- Types of PTMs:
- Glycosylation (糖基化)
- Deamidation (脱酰胺)
- Oxidation
- Acylation (乙酰化)
- PEGylation (PEG, Polyethylene Glycol, 聚乙二醇)
Notes for the edx course Making Biologic Medicines for Patients: The Principles of Biopharmaceutical Manufacturing